
HIPEC for recurrent ovarian cancer.
Study Title: Hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer (CHIPOR): a randomised, open-label, phase 3 trial
Published In: The Lancet Oncology, November 2024
DOI: 10.1016/S1470-2045(24)00531-X
Study Acronym: CHIPOR
✍️ Why This Matters
HIPEC for recurrent ovarian cancer.
Recurrent ovarian cancer, particularly of the high-grade serous or endometrioid subtype, remains one of the most challenging gynecological malignancies to manage. Despite advances in systemic therapies, most patients relapse, often with disease confined to the peritoneum. Cytoreductive surgery has proven benefit, but the role of hyperthermic intraperitoneal chemotherapy (HIPEC) in the recurrent setting has been unclear.
The CHIPOR trial fills this gap as the first randomised phase 3 study to assess whether adding cisplatin-based HIPEC to surgery improves outcomes in patients with platinum-sensitive recurrent ovarian cancer.
🎯 Study Objectives
To determine if the addition of HIPEC (cisplatin 75 mg/m² at 41°C for 60 minutes) to complete secondary cytoreductive surgery improves overall survival (OS) in women with first platinum-sensitive relapse of epithelial ovarian cancer.
🧪 Study Design Snapshot
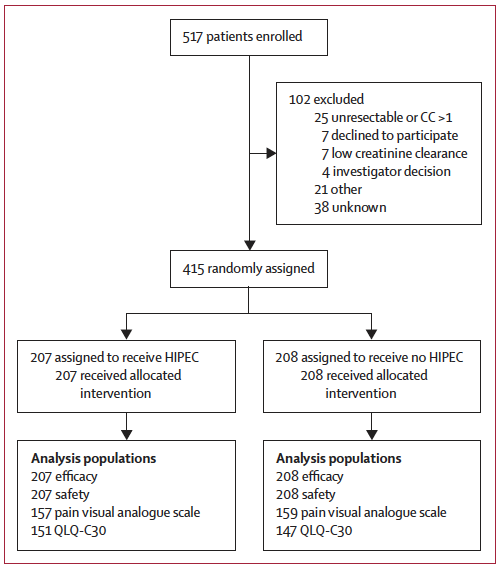
- Design: Multicentre, open-label, phase 3 randomised controlled trial
- Sites: 31 centres across France, Belgium, Spain, and Canada
- Participants: 415 women with first recurrence ≥6 months after initial platinum-based chemotherapy
- Intervention: Surgery with or without single-session HIPEC administered intraoperatively
- Randomisation: 1:1 during surgery, after confirming complete macroscopic resection (CC0/CC1)
Stratification Factors:
- Study centre
- Completeness of cytoreduction (CC0 vs CC1)
- Platinum-free interval (6–12, 12–18, or >18 months)
- Planned use of PARP inhibitors
📊 Key Outcomes
🏁 Primary Endpoint: Overall Survival (OS)
| Group | Median OS | HR (95% CI) | p-value |
|---|---|---|---|
| HIPEC | 54.3 months | 0.73 (0.56–0.96) | 0.024 |
| No HIPEC | 45.8 months | – | – |
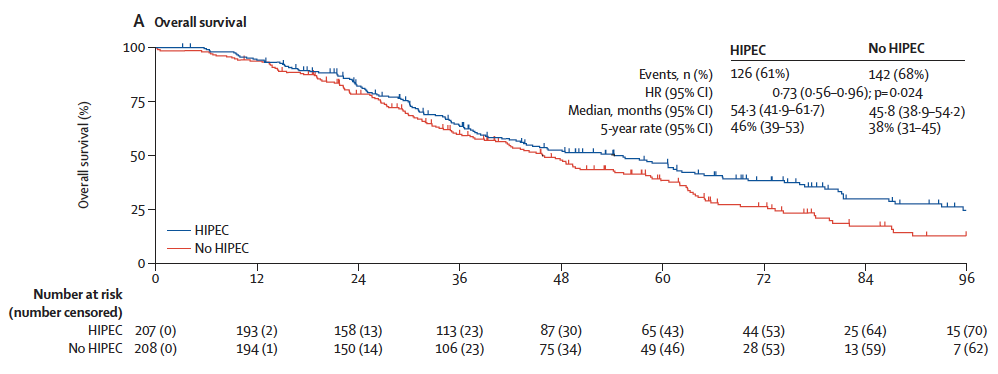
✅ 27% relative reduction in the risk of death in the HIPEC group
✅ 8.5-month survival gain
⏱ Secondary Outcomes
| Outcome | HIPEC | No HIPEC | HR (95% CI) |
|---|---|---|---|
| Progression-Free Survival (PFS) | 10.2 mo | 9.5 mo | 0.79 (0.63–0.99) |
| Peritoneal PFS | 12.3 mo | 12.1 mo | 0.74 (0.57–0.94) |
| Extraperitoneal PFS | 87.8 mo | 86.9 mo | 0.83 (0.59–1.17) |
| Time to Next Therapy | 11.5 mo | 11.4 mo | 0.65 (0.51–0.83) |
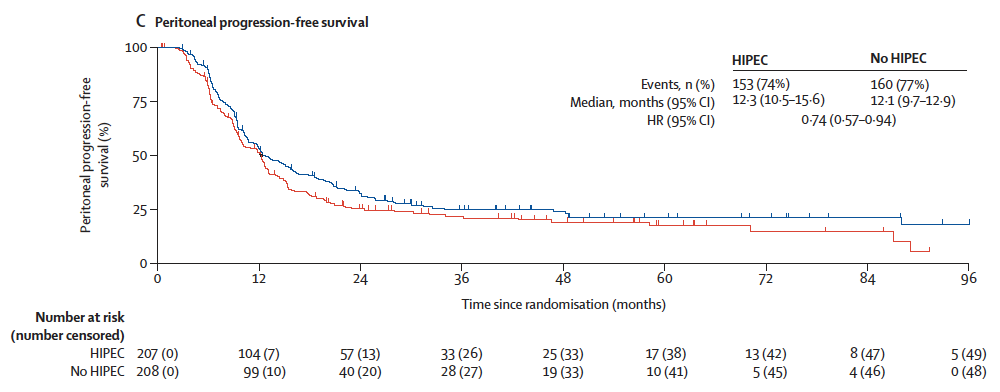
📌 Greatest HIPEC benefit observed in peritoneal PFS, aligning with its locoregional mechanism of action.
🛑 Adverse Events and Renal Protection
Grade ≥3 Adverse Events (within 60 days post-op):
| Event | HIPEC | No HIPEC |
|---|---|---|
| Any Grade ≥3 AE | 49% | 27% |
| Anaemia | 23% | 14% |
| Renal failure | 10% | 1% |
| Electrolyte disturbances | 14% | 1% |
Three postoperative deaths occurred in the no-HIPEC group; none in the HIPEC group.
💡 The Role of Sodium Thiosulfate
Cisplatin’s nephrotoxicity is a major concern during HIPEC. In 2018, the CHIPOR protocol was amended to allow sodium thiosulfate, a nephroprotective agent that neutralizes free platinum ions and reduces tubular damage.
After this amendment:
- Grade ≥3 renal failure dropped from 12% to 2%
- ICU stay reduced from 7 to 6 days
- Hospitalization shortened from 16 to 14 days
- No similar changes were observed in the no-HIPEC group
Bottom line: Sodium thiosulfate should be considered standard adjunctive therapy in cisplatin-based HIPEC protocols.
👁️🗨️ Subgroup Findings
- HIPEC benefit was consistent across age, cytoreduction status, platinum-free interval, and histologic subtypes
- In the CC1 subgroup (incomplete resection), benefit appeared more pronounced
- Patients with BRCA mutations (70% of whom received PARP inhibitors) showed less clear benefit, possibly due to small sample size or competing treatment effects
❤️ Patient-Reported Outcomes
- No significant differences in pain scores or quality of life (EORTC QLQ-C30) between groups
- Pain increased briefly post-surgery but returned to baseline within 3–6 months in both arms
- HIPEC did not worsen patient experience, despite higher acute toxicity
🧠 Clinical Takeaways
- HIPEC offers a significant survival benefit in patients undergoing complete cytoreduction for first platinum-sensitive relapse
- Toxicities are manageable, especially with prophylactic thiosulfate and proper perioperative care
- Best suited for delivery in specialised centres with HIPEC expertise
- Supports broader use of HIPEC beyond the primary setting, aligning with earlier OVHIPEC-1 trial findings
📌 Bottom Line
For patients with a late first relapse of high-grade ovarian cancer amenable to complete cytoreductive surgery, platinum-based HIPEC improves survival and should be considered—ideally in centres with expertise in surgical oncology and renal protection protocols.
🧾 Conclusion
The CHIPOR trial adds high-level evidence to a growing body of literature supporting HIPEC in ovarian cancer. While the technique requires multidisciplinary coordination and careful patient selection, it offers a clear survival advantage without compromising quality of life—an achievement that few treatments in this space can claim.
Incorporating sodium thiosulfate as a nephroprotective agent has made the regimen safer and more feasible for wider adoption. These results should prompt clinicians and surgical oncologists to re-evaluate current standards of care in platinum-sensitive relapsed ovarian cancer and consider referring suitable patients to experienced HIPEC centres.
📣 Call to Action
Are you treating patients with recurrent ovarian cancer?
🔍 Revisit your referral network.
🏥 Connect with HIPEC-trained centres.
🧠 Educate your team on renal protection protocols like sodium thiosulfate.
🎯 Join the Peritoneo.life hub — our dedicated space for brazilian clinicians working on the front lines of ovarian and peritoneal cancers.
📥 Stay ahead—subscribe to p.life papers for monthly evidence-based insights, written for doctors who want clarity, not clutter.

